You have decided to do an IPD Meta-Analysis. What should you do next?
Overview
IPD meta-analyses are major research projects that typically take two years or more to complete. It is therefore important to establish whether undertaking an IPD meta-analysis is feasible. Consider:
- Has a clear, focused research objective been formulated for the IPD meta-analysis?
- Can the right team with the right skills be established?
- How will the IPD be accessed, transferred and stored?
- Can the required funding be obtained and resource (e.g. data manager, statistician) be put in place?
The information included within this section will help you understand the steps involved in undertaking an IPD meta-analysis, and provides some tips and resources to help your IPD meta-analysis run as smoothly as possible!
Steps and Timeline
There are multiple steps involved in undertaking an IPD meta-analysis, which are shown in the figure below. These can be considered as: a) project planning and b) conduct and analysis.
More detail about these specific stages can be found on this website, and via the links below:
- Cochrane methods - frequently asked questions
- Obtaining and managing data sets for individual participant data meta-analysis: scoping review and practical guide
- Meta-analysis of individual participant data: rationale, conduct, and reporting
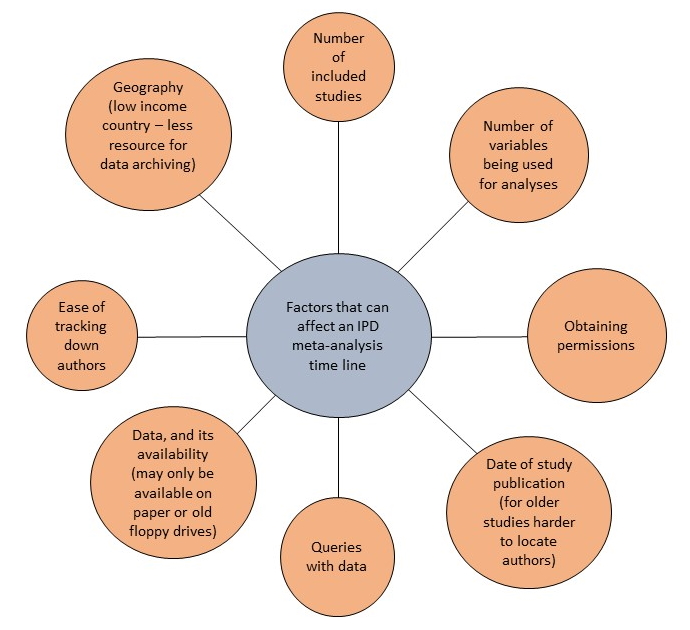
IPD meta-analyses can typically take between 2 and 3 years to complete, sometimes even longer. Factors that might affect the timeline are shown below:
Study selection
This page gives some tips for identifying studies to include in your IPD Meta-Analysis project.
Develop a search strategy
- We recommend that you collaborate with a research librarian to ensure the best quality search
- Search strategies should include all relevant databases in the particular clinical area (e.g. MEDLINE, EMBASE, Web of Science, PsycINFO, Scopus, Cochrane Library)
- Search strategies can be designed by considering subject headings and keywords, and when appropriate, incorporating existing search hedges
- Keep in mind that in contrast to a conventional meta-analysis, you are searching for eligible datasets and not eligible publications. Thus, a study need not report all relevant parameters of interest, so long as the desired information can be obtained from the primary dataset
- Once developed, the search should be peer-reviewed by another librarian
In addition to the formal search strategy, we also recommend:
- Searching relevant grey literature (e.g. trial registries, conference proceedings, theses and dissertations) for the existence of relevant datasets
- Contacting experts (including the corresponding authors of included studies) to inquire about additional relevant datasets from ongoing or unpublished studies
- Hand searching the reference lists of relevant reviews
Here are some resources related to managing citations (post search):
Obtaining data
Do take sufficient time to obtain data from other institutions and organisations. Sharing of data is strongly encouraged by research funders, but in most countries this will require a legal document to ensure that the processes meet with national and international data protection laws (e.g. GDPR in Europe). A data-sharing agreement will describe the aim and methods of the project, ensure all approvals are in place, issues related to Intellectual Property are agreed, and secure processes for transfer, storage, handling, and analysis are used.
Below you will find an example (template) of a data-sharing agreement, which can be adapted in discussion with each institution’s legal team.
- Data sharing agreement example (PANDA-S)
Here are some examples of websites that allow secure data transfer, so that datasets do not need to be emailed. The costs of these services will vary, and so may the level of data security. Your manager or supervisor may be able to advice you as to which of these services are approved and supported by your institution.
Ethics
It is important to establish what, if any, form of ethical approval is required to complete an IPD meta-analysis project. Whilst it could be argued that research ethical approval is exempt as no new data are being provided, host institutions may take different approaches. In addition, whilst study investigators do not generally require formal ethical approval to share their IPD, they may need formal institutional approval. Requirements will vary internationally, institutionally, and are subject to change.
If ethical approval is not required, it is important to state this, as in the example below:
"As this study involved secondary analysis of anonymised previously collected data, the Research Ethics Committee of the ___ declared that this project did not require research ethics approval. However, for each included dataset, we confirmed that the original study received ethics approval and that all patients provided informed consent."
Principles of data sharing
Guidance on good practice principles for sharing IPD from publicly funded clinical trials can be found here:
- Good Practice Principles for Sharing Individual Participant Data from Publicly Funded Clinical Trials (Methodology Hubs PDF)
- Data Sharing Statements for Clinical Trials: A Requirement of the International Committee of Medical Journal Editors (ICMJE.org PDF)
Protocol
As with any major research project, a detailed protocol should be developed stating the aims and objectives, study inclusion and exclusion criteria, methodological approach to be taken, and planned analyses. Example protocols are provided below for various types of IPD meta-analyses.
Investigation of prognostic factors
- Example - prognostic factors for adults with depression in primary care
- Example - external validation of prediction models for pre-eclampsia
Investigation of diagnostic accuracy
- Example - protocol for DEPRESSD-PHQ-9 (diagnostic accuracy IPDMA)
Investigation of intervention effect
- Example - effectiveness of internet-based cognitive behavioural therapy to reduce suicidal ideation and behaviours
Investigation of predictors of treatment effect
- Example – subgrouping and targeting exercise for osteoarthritis (STEER)