Key interests
.png)
We work on a range of biomaterials for neural applications such as hydrogels, electroactive polymers, clinical grade imaging nanoparticles, transfection grade nanoparticles, neurosurgical grade dural repair materials and polymer nanofibers. We work closely with a wide collaborative network of chemists and industrial partners.

The image shows multiple glial cell types of the nervous system growing on polymer nanofiber guides
The group has a background in the use of bioengineering and tissue engineering approaches as adjunct technologies to enhance stem/precursor cell therapies for regenerative neurology, including clinical transplant populations. Key interests include encapsulation of transplant populations in polymer matrices for protected cell delivery and novel engineering methods for stem cell imaging and genetic engineering. We have expertise in the derivation/maintenance of a wide range of neural cells in vitro including neural stem cells, oligodendrocyte precursors and oligodendrocytes, olfactory ensheathing cells, astrocytes and microglia. We also hold a Home Office Licence for in vivo models of neurological injury.
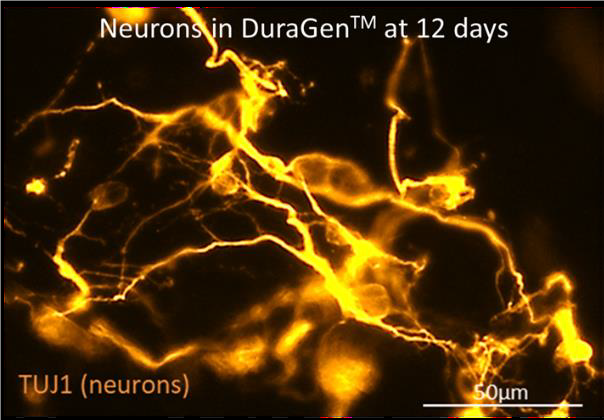
The image shows stem cell derived neurons growing in a neurosurgical grade biomaterial used widely in surgical procedures.
Our interests lie in the use of nanoparticle platforms for neural imaging and genetic modification of neural cells. Specifically, we have an interest in the development and testing of multifunctional nanoparticles that offer imaging and transfection capability. A key interest is the use of stealth nanoparticles to evade immune clearance for neural applications, neural cell processing of nanoparticles, and the influence of surface chemistry on these processes.
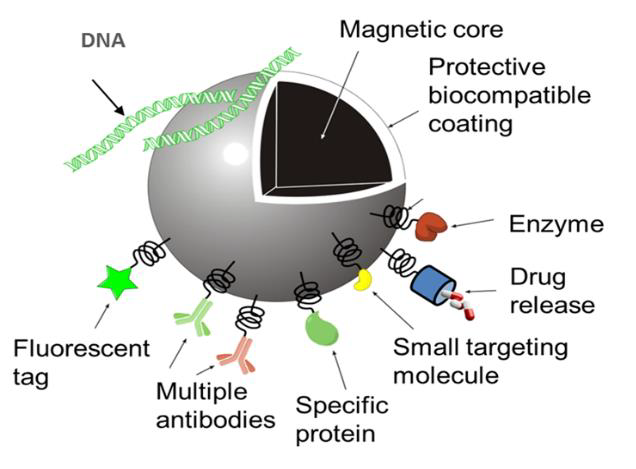
The image is a schematic of the multifunctional properties of iron oxide based nanoparticles for medical applications.
We have a keen interest in developing safe alternatives for genetic modification of neural cells as an alternative to viral systems (adenoviruses, lentiviruses, retroviruses); the latter are highly efficient but are associated with drawbacks for clinical translation such as safety and scale-up for commercial manufacture. We have expertise in the use of Magnetofection technology and testing of novel oscillating field magnetic devices to enhance transfection. A major research interest has been in the use of a novel class of DNA vectors (minicircles) in conjunction with nanoparticles, which offer high promise for safe and sustained gene delivery and high pharmaceutical scale-up potential.
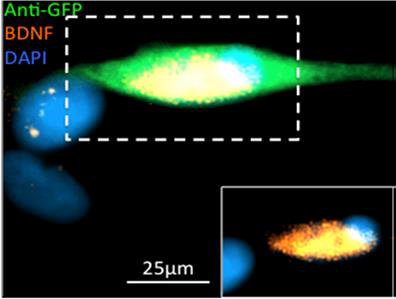
The image shows canine transplant cells derived for a spinal injury transplantation trial that have been genetically engineered to release a therapeutic protein.
Cell culture methods have seen a paradigm shift towards the use of biomimetic and 3D culture systems for experimental research. We have a high interest and expertise in the use of 3D organotypic models with injury paradigms and development of 3D multicellular neural constructs on soft materials tailored to the native stiffness of the nervous system. Investigating the use of the chick embryo in these models for testing of novel nanotherapies has been a major recent development.

The image shows an ‘organotypic’ slice of rodent spinal cord growing in a dish with high cell survival.
We have an interest in the labelling of transplant populations with clinical and experimental grade nanoparticles for non-invasive tracking of stem cells in the nervous system post-grafting. We have particular expertise in developing methods for non-invasive tracking of stem cells encapsulated in polymer matrices.
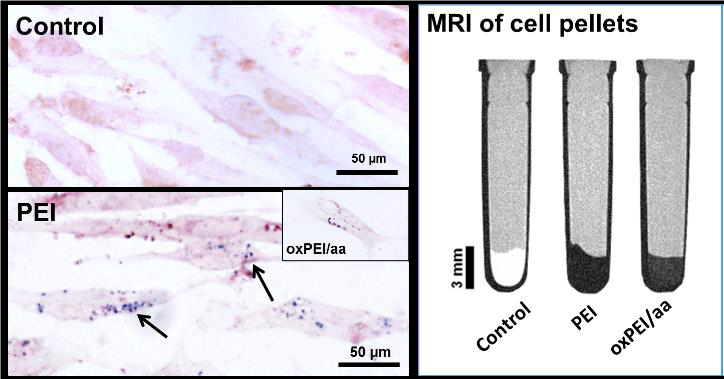
The images shows labelling of stem cells with a novel nanoparticle synthesised in the laboratory of our Israeli collaborator, and its use in cell imaging by Magnetic Resonance Imaging.
We are interested in soft, electroactive materials (e.g conductive and piezoelectric polymers) which could be implanted into the injured nervous system, for electrical stimulation of neural injury sites versus metal based electrodes. We are also investigating whether combined encapsulation in a protective matrix and delivery of electrical stimulation could improve repair by transplant cell populations.
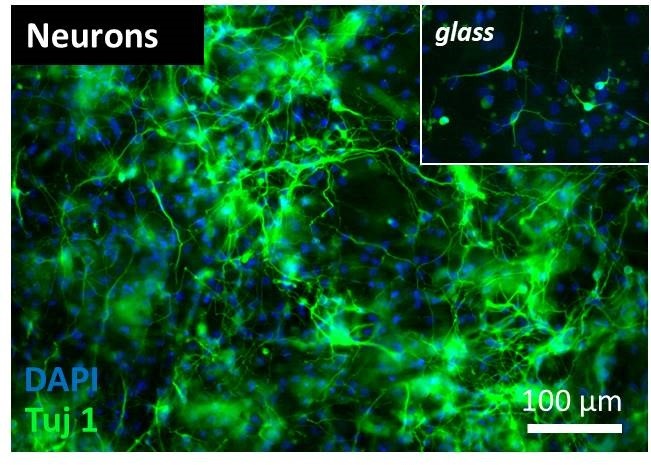
The image shows a dense network of neurons following differentiation of neural stem cells on a 3D conductive polymer scaffold. Neurons appear to comparatively lower branching on standard, glass substrates (inset).


